Intraosseous Device Market is anticipated to register a CAGR of nearly 7.2%, during the forecast period.
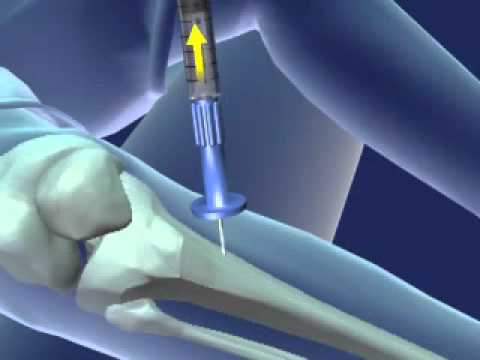
The intraosseous device market refers to the market for devices used to access the intraosseous space, which is the marrow cavity of bones, for the administration of fluids, medications, and other substances directly into the bloodstream. Intraosseous devices provide a rapid and reliable alternative route for vascular access when traditional intravenous access is difficult or not feasible.
Key factors and trends in the intraosseous device market include:
- Increasing Awareness and Adoption: There is growing awareness among healthcare professionals about the benefits of intraosseous access in emergency situations, such as cardiac arrest, trauma, and pediatric emergencies. The adoption of intraosseous devices is increasing as they offer a quick and effective means of vascular access when time is critical.
- Emergency Medicine Applications: Intraosseous devices are commonly used in emergency medicine settings, including pre-hospital care, emergency departments, and critical care units. They provide a rapid means of administering life-saving fluids, medications, and blood products when intravenous access is challenging or delayed.
- Pediatric Applications: Intraosseous devices are particularly valuable in pediatric emergencies, where establishing intravenous access may be more challenging due to small veins and limited patient cooperation. The use of intraosseous devices allows for rapid resuscitation and administration of medications in critically ill or injured children.
- Technological Advancements: The intraosseous device market has witnessed technological advancements aimed at improving ease of use, reducing procedure-related complications, and enhancing patient comfort. Advances include the development of automatic intraosseous devices with adjustable insertion depths, improved needle designs, and enhanced safety features.
- Training and Education Initiatives: Training and education programs have been instrumental in increasing the adoption of intraosseous devices among healthcare professionals. Organizations and institutions offer courses and simulations to educate healthcare providers on proper technique, indications, and complications associated with intraosseous access.
- Military and Disaster Response Applications: Intraosseous devices have gained importance in military and disaster response settings, where rapid and reliable vascular access is crucial in challenging environments. These devices are included in emergency medical kits and are utilized in the field to provide immediate medical care.
- Regulatory Considerations: Regulatory bodies, such as the U.S. Food and Drug Administration (FDA), provide guidelines and standards for the design, manufacturing, and use of intraosseous devices. Compliance with these regulations ensures the safety and effectiveness of the devices and influences market dynamics.
- Product Innovations and Market Competition: Market players are focusing on product innovations to enhance the safety, efficacy, and user-friendliness of intraosseous devices. Companies are investing in research and development to introduce advanced features, such as real-time confirmation of needle placement and integrated electronic monitoring systems. This leads to market competition and the introduction of new devices with improved functionality.
Get Free Exclusive PDF Sample Copy of This Research Report https://stringentdatalytics.com/sample-request/intraosseous-device-market/3339/
Market Segmentations:
Global Intraosseous Device Market: By Company
• PerSys Medical
• Allied Medical
• Teleflex
• Pyng Medical
Global Intraosseous Device Market: By Type
• Intraosseous Needles
• Intraosseous Infusion Device
• Other
Global Intraosseous Device Market: By Application
• Hospitals
• Ambulatory Surgical Centres
Global Intraosseous Device Market: Regional Analysis
All the regional segmentation has been studied based on recent and future trends, and the market is forecasted throughout the prediction period. The countries covered in the regional analysis of the Global Intraosseous Device market report are U.S., Canada, and Mexico in North America, Germany, France, U.K., Russia, Italy, Spain, Turkey, Netherlands, Switzerland, Belgium, and Rest of Europe in Europe, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, China, Japan, India, South Korea, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), and Argentina, Brazil, and Rest of South America as part of South America.
For more information about this report visit:- https://stringentdatalytics.com/reports/intraosseous-device-market/3339/
About Stringent Datalytics
Stringent Datalytics offers both custom and syndicated market research reports. Custom market research reports are tailored to a specific client’s needs and requirements. These reports provide unique insights into a particular industry or market segment and can help businesses make informed decisions about their strategies and operations.
Syndicated market research reports, on the other hand, are pre-existing reports that are available for purchase by multiple clients. These reports are often produced on a regular basis, such as annually or quarterly, and cover a broad range of industries and market segments. Syndicated reports provide clients with insights into industry trends, market sizes, and competitive landscapes. By offering both custom and syndicated reports, Stringent Datalytics can provide clients with a range of market research solutions that can be customized to their specific needs.
Contact Us
Stringent Datalytics
Contact No- +1 346 666 6655
Email Id- sales@stringentdatalytics.com
Web- https://stringentdatalytics.com/
Leave a Reply