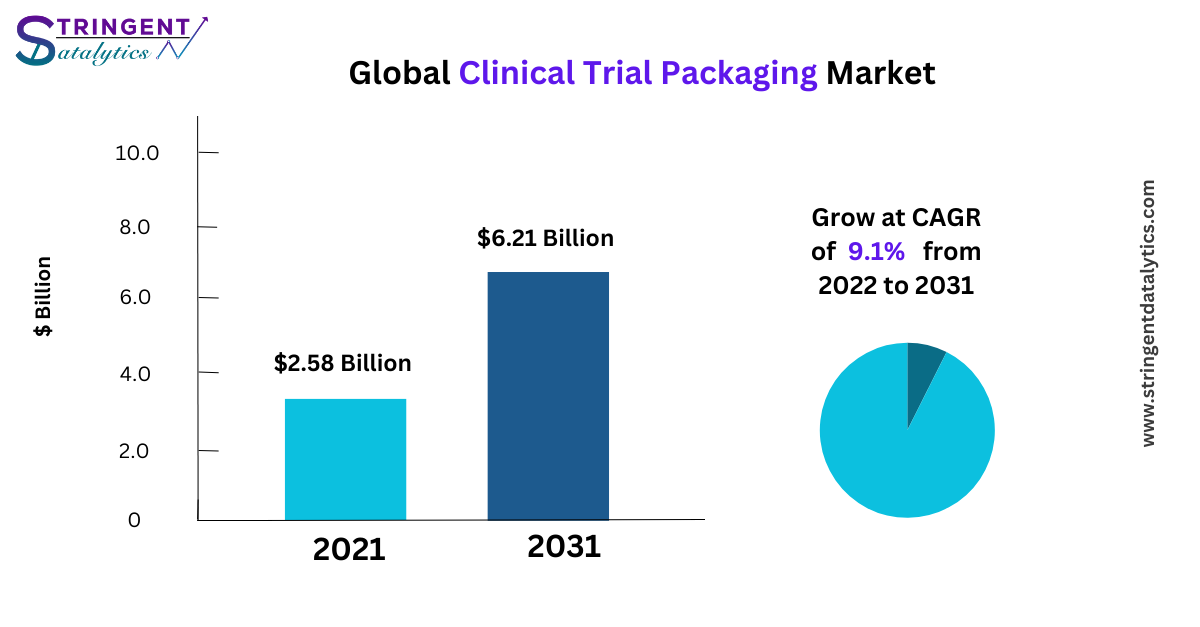
Clinical Trial Packaging Market size was valued at $2.58 Billion in 2021, and is projected to reach $6.21 Billion by 2031, growing at a CAGR of 9.1% from 2022 to 2031.
The Clinical Trial Packaging Market has been experiencing significant growth, driven by the increasing number of clinical trials, stringent regulatory requirements, and advancements in packaging technologies. Clinical trial packaging involves the packaging, labeling, and distribution of investigational drugs and medical devices used in clinical trials. This market plays a crucial role in ensuring the safety, integrity, and compliance of clinical trial materials from the point of manufacture to the trial site and ultimately to the patient.
Key segments within the clinical trial packaging market include:
- Packaging Types:
- Blisters: Commonly used for oral solid dosage forms, blisters provide protection and are easy to use, offering a secure and tamper-evident packaging solution.
- Bottles: Used for liquid formulations, capsules, and tablets, bottles offer versatility and protection against environmental factors.
- Sachets: Suitable for powders, granules, and small-volume liquids, sachets are lightweight and easy to transport.
- Syringes and Vials: Essential for injectable formulations, syringes, and vials ensure sterility and accurate dosing.
- Ampoules: Used for single-dose liquid formulations, ampoules provide excellent protection and sterility.
- Services:
- Labeling: Customized labeling solutions to ensure compliance with regulatory requirements and accurate information dissemination.
- Distribution: Specialized logistics services to manage the complex supply chain of clinical trial materials, including temperature-controlled transportation and real-time tracking.
- Randomization and Blinding: Services to ensure unbiased trial results through randomization and blinding of investigational products.
- Regulatory Compliance: Ensuring that packaging and labeling meet the stringent requirements of regulatory agencies like the FDA, EMA, and other global health authorities.
- End Users:
- Pharmaceutical and Biotechnology Companies: Major users of clinical trial packaging services for drug development and testing.
- Clinical Research Organizations (CROs): Organizations that manage clinical trials on behalf of pharmaceutical companies often rely on specialized packaging solutions.
- Research Institutions: Academic and research institutions conducting clinical trials also require compliant packaging solutions.
Market Drivers and Trends:
- Increasing Number of Clinical Trials: The growing prevalence of chronic diseases, the emergence of new therapeutic areas, and the need for personalized medicine are driving the number of clinical trials globally, boosting demand for clinical trial packaging.
- Stringent Regulatory Requirements: Regulatory agencies impose strict guidelines on the packaging and labeling of investigational products to ensure patient safety and data integrity, necessitating specialized packaging solutions.
- Advancements in Packaging Technologies: Innovations in packaging materials, designs, and technologies, such as tamper-evident features, smart packaging, and eco-friendly materials, are enhancing the efficiency and compliance of clinical trial packaging.
- Growth in Biologics and Biosimilars: The increasing development of biologics and biosimilars, which often require specialized packaging solutions to maintain stability and efficacy, is driving market growth.
- Outsourcing Trends: Pharmaceutical companies are increasingly outsourcing clinical trial packaging to specialized service providers to focus on core research and development activities and to leverage the expertise of packaging specialists.
Challenges:
- Complex Supply Chain Management: The logistics of clinical trial materials can be complex, involving multiple stakeholders, regulatory requirements, and the need for precise temperature control and tracking.
- Cost Constraints: The high cost of specialized packaging solutions and compliance with regulatory standards can be a barrier, especially for smaller companies and academic institutions.
- Regulatory Variability: Different regulatory requirements across countries and regions can complicate the packaging and labeling process, requiring customized solutions and expertise in global regulations.
Future Outlook: The clinical trial packaging market is poised for continued growth, driven by the increasing complexity of clinical trials, the development of new therapeutic modalities, and the ongoing need for compliance with stringent regulatory standards. Technological advancements in packaging, the growing importance of patient-centric designs, and the expansion of clinical trials in emerging markets will further contribute to market expansion. Collaboration between pharmaceutical companies, CROs, and packaging service providers will be essential to address challenges and meet the evolving needs of clinical trial logistics and packaging.
Get Free Exclusive PDF Sample Copy of This Research Report https://stringentdatalytics.com/sample-request/clinical-trial-packaging-market/16358/
Market Segmentations:
Global Clinical Trial Packaging Market: By Company
Bilcare
Fisher Clinical Services
WuXi AppTec
PCI Pharma Services
Almac Group
PharMaterials
PAREXEL
Schreiner MediPharm
Sharp Packaging
The Coghlan Group
Rubicon
Westrock
Xerimis
Catalent
Piramal Pharma Solutions
Corden Pharma
DMB Consultancy
Körber Medipak Systems
Sentry BioPharma
NextPharma
Mawdsleys
Global Clinical Trial Packaging Market: By Type
Syringes
Vials and Ampoules
Blisters
Tubes
Bottles
Bags and Pouches
Sachets
Kits or Packs
Others
Global Clinical Trial Packaging Market: By Application
Drug Manufacturing Companies
Research Laboratories
Clinical Research Organizations
Global Clinical Trial Packaging Market: Regional Analysis
The regional analysis of the global Clinical Trial Packaging market provides insights into the market’s performance across different regions of the world. The analysis is based on recent and future trends and includes market forecast for the prediction period. The countries covered in the regional analysis of the Clinical Trial Packaging market report are as follows:
North America: The North America region includes the U.S., Canada, and Mexico. The U.S. is the largest market for Cold-chain Pharma in this region, followed by Canada and Mexico. The market growth in this region is primarily driven by the presence of key market players and the increasing demand for the product.
Europe: The Europe region includes Germany, France, U.K., Russia, Italy, Spain, Turkey, Netherlands, Switzerland, Belgium, and Rest of Europe. Germany is the largest market for Cold-chain Pharma in this region, followed by the U.K. and France. The market growth in this region is driven by the increasing demand for the product in the automotive and aerospace sectors.
Asia-Pacific: The Asia-Pacific region includes Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, China, Japan, India, South Korea, and Rest of Asia-Pacific. China is the largest market for Cold-chain Pharma in this region, followed by Japan and India. The market growth in this region is driven by the increasing adoption of the product in various end-use industries, such as automotive, aerospace, and construction.
Middle East and Africa: The Middle East and Africa region includes Saudi Arabia, U.A.E, South Africa, Egypt, Israel, and Rest of Middle East and Africa. The market growth in this region is driven by the increasing demand for the product in the aerospace and defense sectors.
South America: The South America region includes Argentina, Brazil, and Rest of South America. Brazil is the largest market for Cold-chain Pharma in this region, followed by Argentina. The market growth in this region is primarily driven by the increasing demand for the product in the automotive sector.
Click Here, To Buy Premium Report https://stringentdatalytics.com/purchase/clinical-trial-packaging-market/16358/?license=single
Reasons to Purchase this Report
- Market segmentation based on qualitative and quantitative analysis, taking into account both economic and non-economic aspects.
- Data on market value (in US dollars) for each section and sub-segment
- Identifies the area and market segment anticipated to experience the quickest growth and hold the majority of the market.
- Analysis by geography showcasing product/service usage in the region and highlighting the market dynamics affecting each region.
- A competitive landscape that takes into account recent service/product launches, collaborations, company expansions, and acquisitions by the companies profiled, as well as the market share of the leading players.
- Comprehensive company profiles for the top players in the industry, including business overviews, corporate insights, product benchmarking, and SWOT analyses
- The industry’s future market forecast in light of recent changes, including growth possibilities, drivers of growth, and obstacles present in both developing and emerging economies.
- Comprises a thorough examination of the market from a number of angles using Porter’s Five Forces analysis.
- Offers market knowledge across the Value Chain
- The current market dynamics scenario and future market expansion prospects
About Stringent Datalytics
Stringent Datalytics offers both custom and syndicated market research reports. Custom market research reports are tailored to a specific client’s needs and requirements. These reports provide unique insights into a particular industry or market segment and can help businesses make informed decisions about their strategies and operations.
Syndicated market research reports, on the other hand, are pre-existing reports that are available for purchase by multiple clients. These reports are often produced on a regular basis, such as annually or quarterly, and cover a broad range of industries and market segments. Syndicated reports provide clients with insights into industry trends, market sizes, and competitive landscapes. By offering both custom and syndicated reports, Stringent Datalytics can provide clients with a range of market research solutions that can be customized to their specific needs.
Contact Us
Stringent Datalytics
Contact No- +1 346 666 6655
Email Id- sales@stringentdatalytics.com
Web- https://stringentdatalytics.com/



Leave a Reply